Chemical bonding
Every molecule is a combination of elements held together by chemical bonds. We need to understand these bonds to predict how molecules form and interact with one another. There are two main kinds of chemical bonds: ionic and covalent.
Ionic Bonding
Ions are atoms that have an electrostatic charge (+ or -) from either losing or gaining electrons. If an atom loses electrons it will become a cation (+ charge), and if it gains electrons it will become an anion (- charge). Opposites attract, so cations and anions will pull each other together. This is called Coulombic attraction, and it is how ionic bonds form. For example, sodium chloride (NaCl) is an ionically bonded molecule:
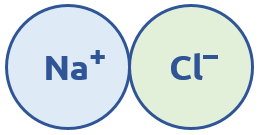
We normally need to have the same amount of positive charge as we do negative charge in an ionic bond. In NaCl, there was one +1 and one -1 charge, so the + and – are evenly distributed. Another example is MgO, where we have a +2 and -2 charge:
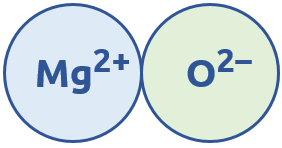
We can also combine more than two ions together to make ionic bonds. For example, MgCl2:
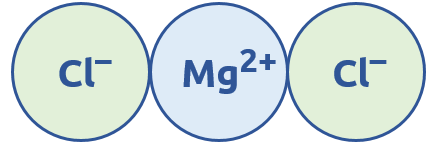
It is necessary for MgCl2 to have two Cl- ions instead of just one because magnesium has a +2 charge. This means it requires two negative charges for the + and – charges to be equal, even if those negative charges are split into two -1 ions.
Covalent Bonding
Covalent bonds are the result of two atoms “sharing” their electrons with each other. The simplest example is H2. Below we can see how two unbonded hydrogen atoms can share their electrons to form a covalently bonded pair:
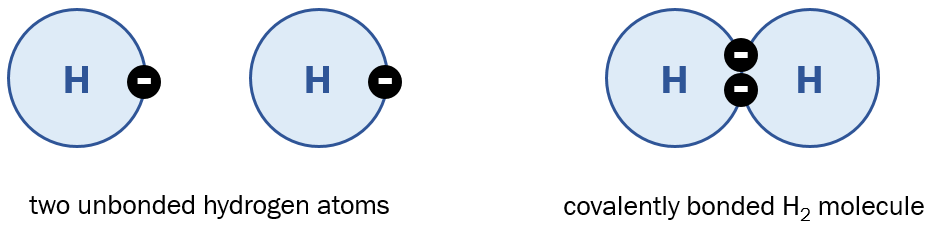
A covalent bond with only two shared electrons is called a single bond, and chemists draw this with a single line connecting the two atoms, like this:
We can also have double bonds (4 shared electrons) and triple bonds (6 shared electrons). Here are some examples of molecules with double and triple bonds, where each line represents 2 bonding electrons:

